Computational insights into cycloadditions of thioisomünchnones with acetylenes: how does sulfur escape from cycloadducts?
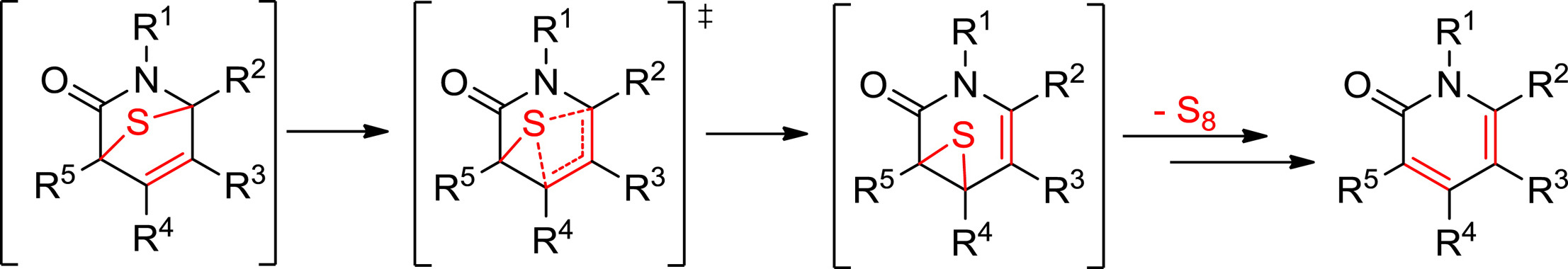
The spontaneous loss of sulfur or isocyanate from transient 7-thia-2-azabicyclo[2.2.1]hept-5-en-3-ones, which are initially formed by 1,3-dipolar cycloadditions of thioisomünchnones with acetylenic dipolarophiles, is the key step in the chemoselective syntheses of pyridin-2-ones or thiophenes. The way by which sulfur is released has been the subject of previous studies pointing to a concerted retro-cheletropic mechanism as a more favorable route than the alternative stepwise pathway. The latter however, is apparently prevalent for elimination of isocyanate. Working with a conformationally-restricted bicyclic thioisomünchnone that undergoes facile cycloaddition with acetylenes, sulfur elimination has now been interrogated by experiment and theoretical calculations at the M06-2X and M11 methods in combination with the 6-311++G(d,p) basis set, which unveil rather a sigmatropic shift via the intermediacy of thiirane species. These results provide new vistas and synthetic opportunities in mesoionic cycloadditions.
- Juan García de la Concepción, Martín Avalos, Reyes Babiano, Pedro Cintas, José L. Jiménez, Mark E. Light, Juan C. Palacios. (2016). Tetrahedron [1] 72 4665-4670.
https://doi.org/10.1016/j.tet.2016.06.041 [2]